The Covid-19 crisis has sparked a truly inspiring wave of citizen-led, open-source innovation, from 3D-printed medical devices and open-source designs for personal protective equipment, to virus tests which could allow for more rapid, large-scale testing. It may well be that the answers to some of the biggest challenges countries around the world are facing are found not in large corporate labs or government research facilities, but in open-source technologist communities, creative collaboration spaces and citizen science labs.
In order to fully take advantage of this wave of creativity and dynamism, however, governments and regulators need to be equally dynamic, both in lifting red tape where needed and in being ready to apply the brakes when necessary.
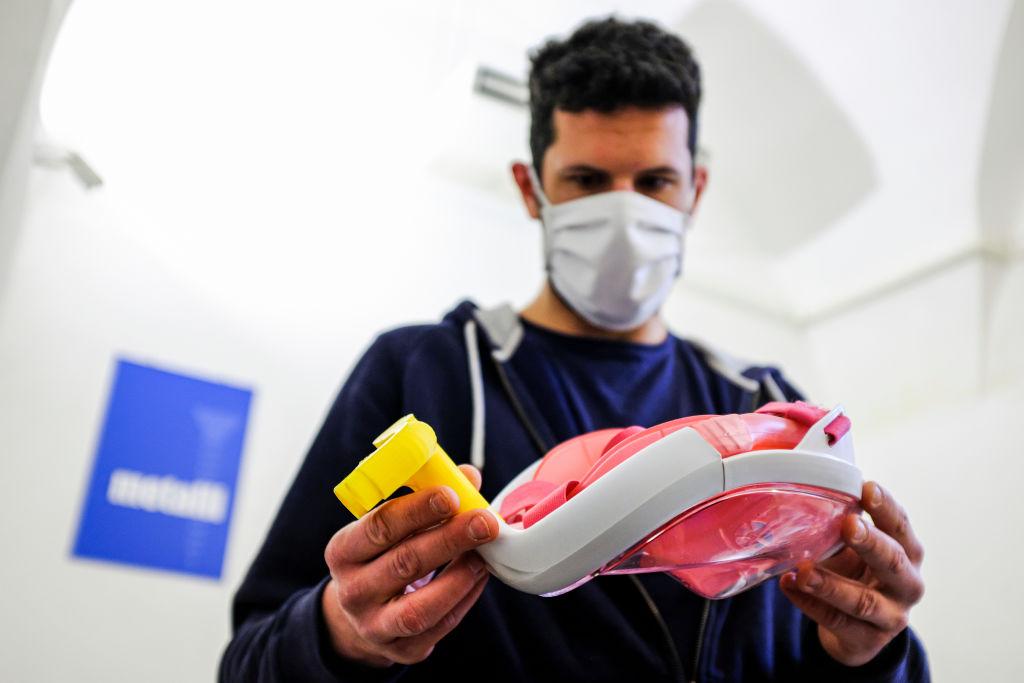
In just a matter of weeks, grassroots open-source projects and international coordination efforts have sprung up around the world to find ways to alleviate the huge shortages of vital equipment exposed by the coronavirus pandemic. In Spain, they’re 3D-printing ventilators; Filipino fashion designers are working with infectious disease specialists to create and share designs for personal protective equipment; maker spaces and artisans are collaborating to rapidly prototype everything from face shields to reusable hospital gowns. In Australia, a community of biohackers in Sydney have adapted an open-source design which could enable more rapid and large-scale testing.
Here’s the problem, though: with only a handful of exceptions, these innovations do not have regulatory approval.
As with so much about this crisis, we’re seeing the dynamics of this push–pull between the critical need for these devices and technology and the equally critical need to ensure that they are safe playing out first in Italy. For example, one Italian start-up has developed a design for a 3D-printed respiratory valve which can be used to adapt snorkel masks into ventilators. Because the device has not been approved, however, patients treated with it are required to sign a declaration acknowledging that they will be treated with an uncertified medical device.
And it’s not just regulators that pose an obstacle. In mid-March, reports surfaced that a company which manufactures and sells ventilator valves for US$11,000 had threatened to sue any volunteers in Italy who had found a way to reproduce the valves for just US$1. The facts of the matter are disputed, but either way it highlights the potential for technology patents to stand in the way of producing vital medical equipment cheaply at scale.
Regulations for medical technology exist for very, very good reasons. As technologist Naomi Wu put it, ‘DIY fucking around with respiration—diving helmets, rebreathers, all that, is right up there with DIY manned flight for huge body count.’ Many innovations spawned during this crisis come from well-intentioned makers with no previous experience building medical devices, and range from ineffective to downright dangerous. Medical professionals and others involved in these efforts are increasingly seeking to hammer home the point that, in this context, the ‘anything is better than nothing’ approach is fundamentally wrong and could result in deaths.
No one wants doctors and patients to have to choose between using boot-strapped, insufficiently tested and potentially dangerous equipment on the one hand, or no equipment at all on the other. Nor do we want to see cases of Covid-19 missed either because we didn’t have enough approved tests or because we used unapproved testing methods which later turn out to be inaccurate. Everything about this crisis involves hard choices, with lost time counted in lost lives.
What that could mean for regulators is a need to operate differently. Responding to the urgent needs of frontline medical staff should involve developing a fast-track regulatory process (as suggested by ASPI’s Michael Shoebridge), but it could also involve engaging much earlier and much more actively with the open-source technologist community.
Producing simple, accessible resources that explain the regulatory requirements for devices like ventilators or equipment like respiratory masks or that set out the process for getting regulatory approval could be one step. Another option for regulators could be working with health authorities to actively scan for and identify the most promising ideas among these grassroots and open-source efforts, and then proactively helping them through the process. Governments could also make it clear that they will come down like a ton of bricks on companies attempting to stand in the way of life-saving innovation in order to bolster their own profits.
Amid the darkness of the Covid-19 pandemic, the energy, enthusiasm and commitment of the individuals and communities around the world who are working together to find solutions is a bright spark. The role of governments and regulators is to catch that spark and magnify it, and use it to help light a way out of this crisis.